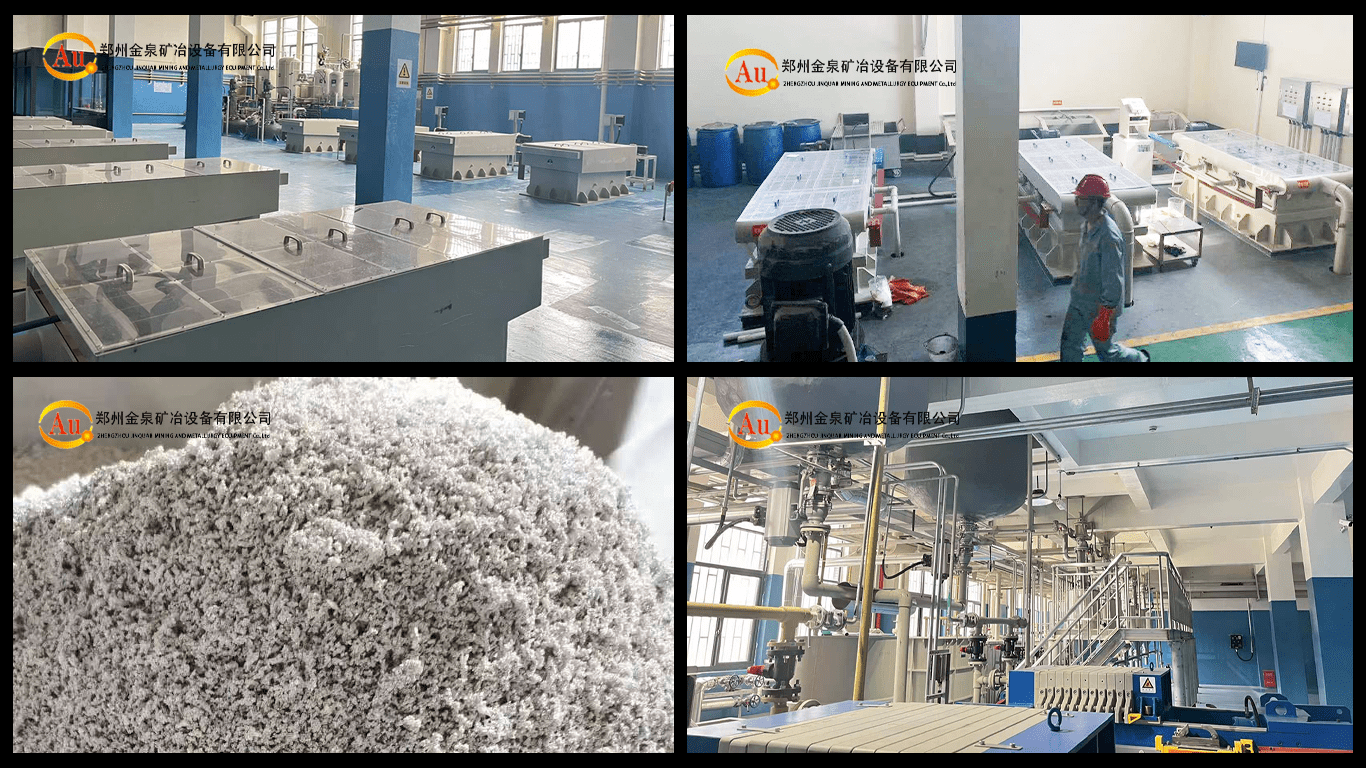
Crude silver electrolysis 9999silver.The raw material of crude silver electrolytic
silver refers to the unrefined silver obtained from the preliminary smelting of
silver ore or silver-containing waste.
Its purity typically ranges from 70% to 99%, and it retains numerous impurities.
Besides silver (Ag) as the main component, crude silver often contains other
precious metals such as gold (Au), platinum (Pt), and palladium (Pd). It also
includes base metal impurities like lead (Pb), copper (Cu), zinc (Zn), nickel (Ni),
and iron (Fe). Moreover, some crude silver features non-metallic impurities—sulfur (S),
arsenic (As), antimony (Sb), and bismuth (Bi)—which originate from ore impurities
or smelting by-products.
To enhance silver purity, we commonly purify crude silver using electrolytic
refining technology. In this process, we use crude silver as the anode and pure
silver sheets as the cathode, while the electrolyte typically consists of silver nitrate (AgNO₃)
and nitric acid (HNO₃). As the electric current passes through, the anode dissolves
silver to produce Ag⁺ ions, and the cathode deposits pure silver, thereby raising the
silver purity to 99.99%. Simultaneously, precious metals like gold, platinum, and
palladium remain undissolved and form anode mud that settles at the bottom of
the electrolytic cell, which we can further recover. In addition, copper and lead
impurities enter the electrolyte and undergo partial removal as we adjust the
process.
Overall, electrolytic refining not only transforms crude silver into
high-purity 99.99% silver but also enables the comprehensive recovery of precious
metals, thereby enhancing economic benefits. Consequently, mining smelting,
electronic waste recycling, and other industries widely adopt this process, as it
offers an efficient solution for silver refining and resource recycling.
Crude silver electrolysis 9999silver vido
Call us now: